Molecular metal-metal bonds, ghost atoms and electron reservoirs
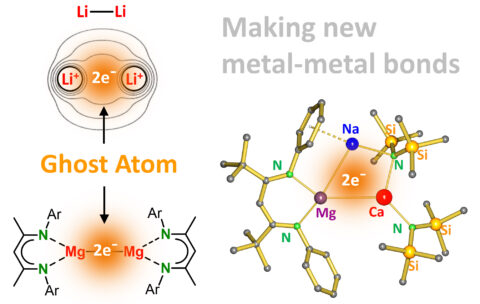
Although bonding and properties of bulk metals are well-understood, creating bonds between only two metals is one of the challenges of modern organometallic research. Molecular metal-metal bonds behave quite differently from bulk metal and have found application in material design or as bimetallic catalysts, for example for the production of hydrogen. While a large variety of bonds between noble, electron-rich transition metals have been studied, there are hardly examples of bonds between electron-poor, electropositive, s-block metals. Dilithium, Li-Li, is the simplest case. In Star Trek Li2 was claimed to be essential as fuel for the spaceship Enterprise. In chemistry classes it is a popular compound to exercise Molecular Orbital theory. In reality, it is highly unstable and a prime example why bonds between electropositive metals are problematic. The Li atoms of very low electronegativity both want to lose their valence electron which end up as “free” electrons between the Li+ nuclei. This maximum in the electron density can be described as a “non-nuclear-attractor”. A ghost atom: there are electrons but there is no nucleus! The first metal-metal bond between electropositive metals has been realized in 2007 for magnesium [1]. This Mg-Mg bond is well-protected by large ligands and its ghost atom acts as an electron reservoir for reducing molecules. Since 15 years, there have been many attempts to tie heavier, even more electropositive metals together. A publication in Nature Synthesis by the Harder group at the FAU now describes a new approach to make molecular entities in which three different s-block metals are bound in a string [2]. This simple “mix and match” method can generate bonds between large varieties of metals and makes use of an unusual complex with anionic Mg [3]. These new trimetallic molecules can be seen as molecular bottles for storage of electrons. By setting these electrons free, the complexes can act as very strong, soluble reducing agents which have already shown their ability to break highly stable molecules. The article is accessible using the link below or can be requested by contacting the authors.
Link: https://shorturl.at/exV25
Contact/Information:
Prof. Dr. Sjoerd Harder, E-mail: sjoerd.harder@fau.de
[1] S. P. Green, C. Jones, A. Stasch, Science 2007, 318, 1754.
[2] J. Mai, J. Maurer, J. Langer, S. Harder, Nat. Synth 2023, https://doi.org/10.1038/s44160-023-00451-y
[3] B. Rösch, T. X. Gentner, J. Eyselein, J. Langer, H. Elsen, S. Harder, Nature 2021, 592, 717.
