Aluminium and iron: a strong team
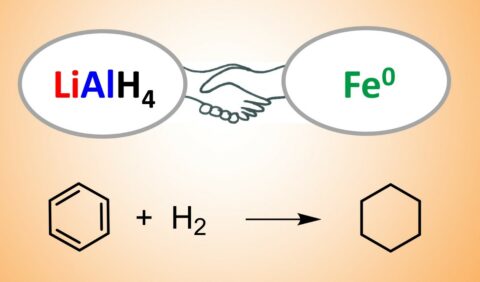
Lithium aluminium hydride (LiAlH4) is a versatile bulk reagent for reduction of ketones to alcohols, or imines to amines. This is a stoichiometric process, needing a lot of LiAlH4 and creating quite some lithium and aluminium waste. Recently the Harder group showed that the imine-to-amine conversion under hydrogen only needs small catalytic quantities of LiAlH4 [1, 2]. It is now found that addition of just a pinch of iron powder transforms LiAlH4 into a catalyst for the challenging reduction of highly stable aromatic molecules like benzene. While iron or LiAlH4 alone do not react with benzene, tiny amounts of the mixture catalyze the conversion of benzene to cyclohexane. Even better, the lithium component is not needed: aluminium/iron combinations are considerably more active for benzene reduction. Such reductions become increasingly important in storing hydrogen for a future energy technology. They are normally most efficiently catalyzed by precious metals like palladium or platinum but combining the two most abundant metals in the world, aluminium & iron, is a sustainable alternative. As previously reported, the secret is to activate the iron by metal evaporation [3].
[1] Nature Catalysis 2018 https://doi.org/10.1038/s41929-017-0006-0
[2] Angewandte Chemie 2018 https://doi.org/10.1002/anie.201803804
[3] Nature Communications 2022 https://doi.org/10.1038/s41467-022-30840-4
Publication
, , , , Angew. Chem. Int. Ed. 2023, e202219016; Angew. Chem. 2023, e202219016. : https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202219016
Contact
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de