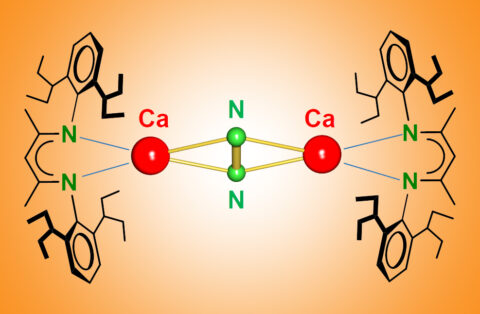
Breaking the N≡N triple bond with calcium
FAU chemists report in Science how to activate nitrogen with the main group metal calcium (Ca)
Molecular nitrogen (N2) is a highly stable gas with a N≡N triple bond that is among one of the strongest known chemical bonds. It reacts with hardly anything and is used as a protective gas for food storage. Being the main component of air, it is also used by plants as a nitrogen source. However, in order to take up nitrogen, the fully inert N2 must first be converted in water soluble ammonium or nitrite and nitrate ions. Plants do this by complicated enzymes but the process is extremely slow. While nature has time, mankind converts N2 to ammonia (NH3) by the Haber-Bosch process. The bulk production of NH3 needs high temperatures and pressures (500 °C, 300 bar). Initially, the process was invented for the production of explosives but nowadays enormous amounts of NH3 are needed for fertilizers to grow crops for a rapidly increasing world population. This industrial “bread from air” process consumes up to 2% of all our energy.
Both, nature and industry, use a transition metal catalyst for N2 conversion. The partially filled d-orbitals on the metal are the key to N2 activation. Now Sjoerd Harder, professor of Inorganic and Organometallic Chemistry, and graduate student Bastian Rösch discovered N2 activation by the main group metal Ca at very low temperatures.
Bastian Rösch: “It was actually a complete surprise to us. Together with my colleague Thomas Gentner we tried to make complexes of Ca in the unusual oxidation state +I. We found that these complexes reduced the aromatic solvents. For instance, benzene became anti-aromatic by picking up two electrons. So we switched to alkanes as solvent and found that Ca(+I) is even able to reduce N2 which we ironically used as a protective gas!”
The Science article can be found at: https://science.sciencemag.org/content/371/6534/1125
Sjoerd Harder: “We found that the activated N2 can be converted to hydrazine (N2H4) which in contrast to N2 is highly reactive and used as rocket fuel. That Ca can do this trick is highly surprising and we started to think that d-orbitals on Ca may be important. Comprehensive calculations by theory groups from Marburg and Nanjing confirmed this possibility. Although highly controversial, this will likely change chemistry text books and maybe initiate industrial research. Our discovery will certainly not replace Haber-Bosch but the fact that the Haber-Bosch catalyst contains not only iron but also calcium oxide is food for thought. Also the presence of Ca in many enzymes or in the Frank-Caro process, the reaction between Ca carbide and N2, may not be coincidental. Time will tell what else Ca can do. Our group will certainly do its best to uncover further secrets of the metal Ca.”
Further information:
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de