Indigo based Molecular 4D Switching in Solution and within Light Responsive Polymers
Molecular switches are molecules whose geometry, chemical, and physical properties can be intentionally altered by an external stimulus. Stimuli include light of defined wavelengths, pH changes, temperature, binding of metal ions, or electric current. The different stimuli and their combinations can drive the switches into distinct and interconvertible geometric or electronic states. Such defined state changes can then be used for applications in e.g. data storage or in the biological/pharmacological context.
Nature provides us with a broad plethora of organic dyes, such as the denim dye Indigo, first synthesized by Adolf von Bayer at the end of the 19th century, or its dibrominated derivative Tyrian purple. Building on these colored natural substances, chemists can create novel functional molecular switches whose geometries and electronic properties are controllable within the Brownian storm of molecular motions.
Conventional molecular switches have two states with different intrinsic energies, geometries and electronic properties. For the subclass of molecular photoswitches, light with defined wavelengths can enrich either the energetically favorable or unfavorable state, while an increase in ambient temperature only accumulates the energetically favorable state.
However, is it possible to have more than two states that can be addressed and controlled? And is there a stimulus, other than light, that can address energetically unfavorable states? Like some kind of chemical fuel?
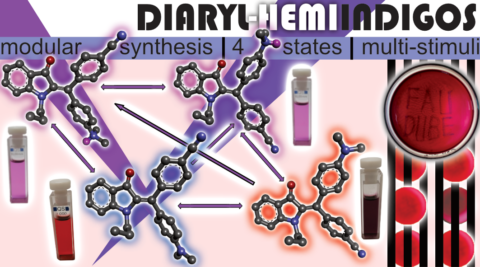
Researchers from the Chair of Organic Chemistry I (Prof. Dr. Henry Dube) at FAU have succeeded in creating a new class of indigo based molecular 4D switches. These new diaryl-hemiindigo switches possess two aryl substituents on the central double bond, allowing them to adopt four different selectively controllable states. The synthesis is modular, involving three to four steps, and allows maximum versatility in terms of structural diversity. Depending on the sequence and combination of the external stimuli green and red light, varying levels of acidity/alkalinity, and temperature, the four molecular states can be independently enriched within up to three different pathways. Green light and acid act as physical and chemical fuels. The four states of the molecular switches are visually distinguishable and can exhibit the colors red, dark violet, or magenta. The strong color contrasts between the different states, their pronounced electronic differences (photochromism), their robustness against different environmental conditions (solvents of varying polarity and solid-state), and their high thermal stability, allow applications in transparent colored polymers. These polymers can be inscribed with light of defined wavelengths. The resulting inscription on a molecular basis can be erased with light of a different wavelength, sunlight, or acid.
The complexity of the stimuli and their sequences, combined with the control over electronic and geometric changes of the colored molecules, offers new possibilities in the development of intelligent materials, data storage, or applications in the field of drug activation through external stimuli.
The results were published in Nature Communications.
Update: The publication has been placed on the Nature Communication website as an Editor’s Highlight. The Editor’s Highlight web page features the top 50 papers recently published in Nature Communication.
Further information:
Publication: Sacherer, M., Hampel, F. & Dube, H. Diaryl-hemiindigos as visible light, pH, and heat responsive four-state switches and application in photochromic transparent polymers. Nat Commun 14, 4382 (2023). https://doi.org/10.1038/s41467-023-39944-x
Contact:
Prof. Dr. Henry Dube
Department of Chemistry and Pharmacy
Chair of Organic Chemistry I
- Phone number: +49 9131 85-65571
- Email: henry.dube@fau.de