Just add a pinch of iron
The group around Prof. Dr. Sjoerd Harder, Chair of Inorganic and Organometallic Chemistry, just reported in Nature Communications a new class of catalysts for hydrogenation, one of the most important industrial processes for the production of bulk or fine chemicals. With smallest catalyst quantities, highly substituted alkenes or even aromatic rings can be efficiently hydrogenated. Especially hydrogenation of aromatic rings normally requires catalysts based on noble metals like platinum. The new hybrid catalyst is a mixture of two abundant metals, iron (Fe) and barium (Ba). It was also found that the addition of Fe turns even more abundant and benign metals like magnesium (Mg) or calcium (Ca) into highly active hydrogenation catalysts.
“The key to this chemistry is the activation of these metals by metal vapor synthesis”, says Prof. Harder. “That means, the metals are evaporated under vacuum and precipitated by rapid cooling in an organic matrix. Our cooperation partner at the Universität Duisburg-Essen (Prof. Dr. Stephan Schulz and Dr. Georg Bendt) found that this gives a very porous material with a large, highly reactive surface area. We have been very lucky that Ulrich Zenneck, who is an emeritus professor at FAU, introduced us to this technique and transferred 40 years of know-how to the Akademische Oberrat Dr. Christian Färber.”
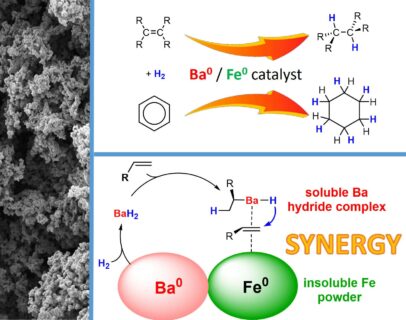
“Previously we already found that activated iron itself is a poor catalyst and, quite unexpected, the main group metal barium is surprisingly active but not great”, Dr. Färber adds. “Then I just mixed the two metals together and found an amazing increase of catalyst activity by at least a factor of 1000! I couldn’t believe it. Instead of a reaction time of 144 hours with the barium catalyst alone, the barium-iron combination fully converted benzene to cyclohexane within half an hour. The proposed mechanism is supported by many observations. Both metals cooperate. The iron surface binds benzene which then is activated for chemical attack by the barium hydride intermediate.”
“These catalysts are unique in the sense that main group metals work together with iron, a cheap and abundant transition metal”, continues Prof. Harder. “Also unique is the cooperation between a homogeneous catalyst in solution and an insoluble heterogeneous catalyst. At the end of the reaction everything precipitates and becomes solid again. Because iron is magnetic, the complete catalyst can be removed simply with a magnet and is used again without loss of activity. That makes this chemistry really sustainable. We are very excited about these new catalysts and feel this is only the start. With so many metals in the periodic table, the number of combinations is nearly endless.”
Further information
Open Access Article in Nature Communications: https://rdcu.be/cPimn
Contact
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de