“Green” Chemistry with Orange Balloons
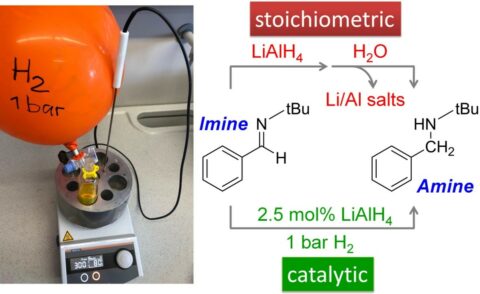
Lithium Aluminium Hydride (LiAlH4) is one of the most potent reducing agents. It is routinely used in organic synthesis for the reduction of unsaturated to saturated bonds. For example, many amines can be prepared from imines by reaction with LiAlH4. Since the reagent needs to be used in larger (stoichiometric) quantities, also large amounts of lithium and aluminium salts are produced as waste. Working with highly reactive LiAlH4 also poses a danger: it can react vigorously and uncontrolled with water which may cause fires. Therefore the reactions are generally run under the inert gas nitrogen. In the last step, however, careful and controlled addition of water is necessary. Despite these shortcomings, industry uses bulk quantities of this important reducing agent.
The research group of Professor Sjoerd Harder, Chair of Inorganic and Organometallic Chemistry at the Department of Chemistry and Pharmacy of FAU, could now show that imine reduction in some cases can simply be done with minute (catalytic) quantities of LiAlH4. When the reaction is run under hydrogen gas instead of under the inert gas nitrogen, only a sniff of LiAlH4 is needed and hardly any lithium and aluminium waste is produced. This “green” chemistry can be done under mild conditions and atmospheric H2 pressure: a simple balloon filled with hydrogen suffices. This method, which attracted world-wide attention, is currently being optimized and extended.
Article: H. Elsen, C. Färber, J. Pahl, G. Ballmann, S. Harder Angew. Chem. Int. Ed. 2018, 57, 7156-7160. https://doi.org/10.1002/anie.201803804
Highlight of this work: D. Schilter Nature Chem. Rev. 2018, 2, 49. https://www.nature.com/articles/s41570-018-0011-0
Contact:
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de