Double Strike for the Alkaline Earth Metal Hydrides
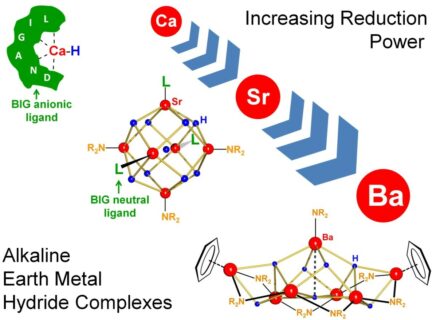
Apart from MgH2, which is widely investigated for its hydrogen storage properties, alkaline earth metal hydrides are generally of no interest. The salt CaH2 reacts vigorously with water but is in contrast to other metal hydrides like LiAlH4 fully inert towards most organics. This is primarily due to its complete insolubility on account of extremely high crystal lattice energies. The research group of Professor Sjoerd Harder PhD, Chair of Inorganic and Organometallic Chemistry of the Department of Chemistry and Pharmacy of FAU earlier showed that soluble calcium hydride complexes, stabilized by a big anionic ligand, are much more reactive and highly useful in catalysis.
Access to the heaviest and even more reactive alkaline earth metal hydride complexes, however, remained barred. Extensive research on the design of big ligands for stabilization of strontium hydride complexes was not successful but a new concept led to the final breakthrough: Instead of using large anionic ligands, large neutral ligands were able to stabilize the first strontium hydride complex. This highly reactive larger cluster was formed by self-assembly, dissolves well in organic solvents and is thermally stable.
The same methodology, however, was not successful for the last remaining challenge: isolation of the largest alkaline earth metal hydride. Surprisingly, the first barium hydride cluster could be isolated without using any larger stabilizing ligands. The cluster, Ba7H7(NR2)7, dissolves very well in organic solvents, is thermally stable and highly reactive. In preliminary reactivity studies even isolated C=C double bonds could be reduced. The extreme reducing power and applications of this first barium hydride cluster in catalysis are currently under investigation.
Reports on these first strontium and barium hydride clusters have in both cases been published as “HOT PAPER” in Germany’s flagship chemistry journal Angewandte Chemie: DOI: 10.1002/anie.201706786 and DOI: 10.1002/anie.201709771.
Contact
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de