The Magnesium-Calcium bond – Very Long and Reactive!
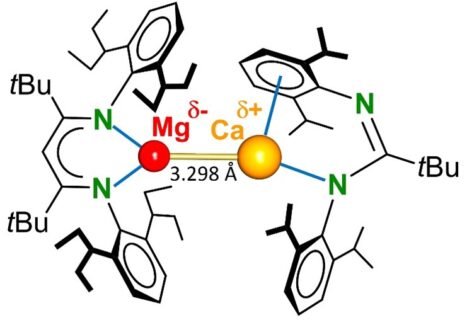
It is already known for 20 years that a group 2 metal like magnesium can not only form Mg+II compounds. Also compounds with Mg+I metal centres have been isolated (Science 2007, 318, 1754). Such compounds typically feature Mg-Mg bonds. All attempts to isolate a similar, but much more reactive, Ca+I complex with a Ca-Ca bond so far failed. The Inorganic Chemistry team around Professor Harder questioned whether mixed-metal Mg-Ca compounds may be stable.
After several failures, now finally a first complex with a Mg-Ca bond has been isolated. The difficulty in such bonding is that both metals are electropositive and do not want to have valence electrons. Exactly this makes such bonds extremely weak and reactive. However, with the right combinations of two different ligands the first Mg-Ca bond could be realized. With circa 3.3 Å, the Mg-Ca bond is among the longest metal-metal bonds recorded. It is nearly of the same length as the bond between two large uranium metals, for example found in U2 trapped inside a C80 fullerene.
Despite this very long Mg-Ca bond, the complex as such is surprisingly stable and only slowly decomposes at room temperature. However, the electronegativity difference between Mg (1.31) and Ca (1.00) creates a polarized Mg(-)-Ca(+) system that even at -80 °C rapidly reduces H2. This bond polarization makes it much more reactive than the unpolarised Mg-Mg bond which does not react with H2. The complex can therefore be regarded as a very strong reducing agent that is soluble in organic solvents.
Such electron transfer processes are fundamental to the chemistry of Mg or Ca batteries.
Further information
Contact
Prof. Dr. Sjoerd Harder
Chair of Inorganic and Organometallic Chemistry